September 10, 2018
Centers for Medicare & Medicaid Services
Department of Health and Human Services
Attention: CMS-1693-P
P.O. Box 8016
Baltimore, MD 21244-8016
RE: CMS-1693-P Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Reporting Program; and Medicaid Promoting Interoperability Program
To Whom It May Concern:
As participants in the Adult Vaccine Access Coalition (AVAC), we appreciate the opportunity to comment on the Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Reporting Program; and Medicaid Promoting Interoperability Program. As discussed further in our letter, AVAC is concerned about the proposed reduction in vaccine administration reimbursement outlined in the rule. We are also concerned about the proposed removal and the Pneumonia Vaccination Status for Older Adults measure (ACO #15) in the Medicare Shared Savings Program (MSSP’s) but greatly appreciate the addition of zoster, pneumococcal and influenza measures in several Alternative Payment Models (APMs) as well as a number of specialty provider measure sets.
AVAC consists of over 50 organizational leaders in health and public health that are committed to addressing the range of barriers to adult immunization and to raising awareness of the importance of adult immunization. AVAC works towards common legislative and regulatory solutions that will strengthen and enhance access to adult immunization across the health care system. Our priorities and objectives are driven by a consensus process with the goal of enabling the range of stakeholders to have a voice in the effort to improve access and utilization of adult immunizations.
One of our key coalition priorities is to advocate for federal benchmarks and quality measures to encourage improved tracking and reporting of immunization status that will result in increased adult immunization rates. In 2016, AVAC released a White Paper outlining the value and imperative of quality measures for adult vaccines.1 The report highlights the role of vaccine quality measures in preventing illness and death, reducing caregiving demands, avoiding unnecessary healthcare spending, and setting the foundation for healthy aging. Quality measurement programs through Medicare play a critical role in promoting improved quality and encouraging adherence to and consistent utilization of recommended adult vaccines.
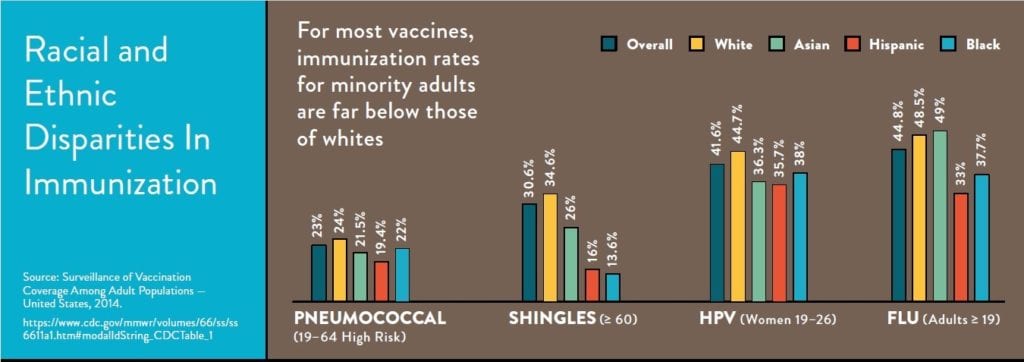
The Department of Health and Human Services (HHS) recognizes that immunization is an important tool to keep people healthy and reduce avoidable health care costs. In its Strategic Plan FY 2018 –2022, HHS acknowledges that “infectious diseases are a major health and economic burden for the United States.2” Additionally, strategic objective 2.1 makes a commitment to “support access to preventive services including immunizations and screenings, especially for high-risk, high-need populations.”2 Unfortunately, access to vaccines is not equal across a person’s lifespan. Despite the well-known benefits of immunizations, more than 50,000 adults die from vaccine-preventable diseases while adult coverage lag behind Healthy People 2020 targets for most commonly recommended vaccines: influenza, pneumococcal, tetanus, hepatitis B, herpes zoster, and HPV.
AVAC believes that adult immunization quality measurement meets the three core strategies underlying the movement toward a truly patient-centered health care delivery system by: 1) Improving the way clinicians are paid to incentivize quality and value of care over simply quantity of services; 2) improving the way care is delivered by providing clinical practice support, data and feedback reports to guide improvement and better decision-making and; 3) making data more available in real-time at the point of contact and enabling the use of certified Electronic Health Record (EHR) technology and other data sources to support care delivery.
The main purpose of the proposed rule is to update payment policies under the physician fee schedule as well as make other changes under Medicare Part B policy. In that context, the proposed rule contains a number of important provisions aimed at the transition from volume-to-value based payment policy. Specifically, the proposed rule includes elements pertaining to the operation of the Medicare Shared Savings Program (MSSP) as well as the Merit-based Incentive Payment System (MIPS). These two programs offer important opportunities to encourage access to and utilization of recommended adult immunizations to priority populations within the Medicare program.
p. 35707 Reduction in Practice Expense Relative Value Units for Vaccine Administration
AVAC is deeply concerned with the proposed reduction in reimbursement rates for CPT codes for vaccine administration due to current gaps in immunization access as well as the potential negative long-term impact on providers’ ability to offer recommended immunizations to Medicare patients.
Immunizations are an important public health imperative and ensuring that immunization providers are properly reimbursed is key to fostering a sustained environment of timely immunization. Vaccine administration by health care providers in their office, at the point of care, is an opportunity that needs to be maintained and encouraged. Studies show that inadequate reimbursement for vaccination administration result in missed immunization opportunities3 and declines in immunization rates. Given HHS’s Healthy People 2020 goals and the gaps in care that current exist in adult vaccination, any reimbursement reductions at the physician/provider level could widen those care gaps and have unnecessary population health consequences.
AVAC urges CMS to maintain CPT codes for vaccine administration at rates that properly reimburse for the cost of the service and will continue to encourage providers to offer Medicare beneficiaries recommended immunizations at the clinical point of care.
p. 35935 Merit-based Incentive Payment System (MIPS) Alternative Payment Model (APM) – Comprehensive ESRD Care
AVAC is pleased the merit-based Incentive Payment System (MIPS) Alternative Payment Model (APM) for comprehensive ESRD Care in the proposed rule includes the following adult immunization measures:
➢ Influenza Immunization for the ESRD Population
➢ Pneumococcal Vaccination Status (NQF #0043)
p. 35939 Merit-based Incentive Payment System (MIPS) Alternative Payment Model (APM) – Comprehensive Primary Care Plus (CPC+) Model
AVAC is pleased the Merit-based Incentive Payment System (MIPS) Alternative Payment Model (APM) for the Comprehensive Primary Care Plus (CPC+) Model in the proposed rule includes the following adult immunization measures:
➢ Preventive Care and Screening: Influenza Immunization (NQF#0041)
➢ Pneumococcal Vaccination Status for Older Adults (NCQA measure)
p. 35945 Merit-based Incentive Payment System (MIPS) Alternative Payment Model (APM) – Maryland Total Cost of Care Model
AVAC is pleased the Merit-based Incentive Payment System (MIPS) Alternative Payment Model (APM) for the Maryland Total Cost of Care Model includes the following adult immunization measures:
➢ Preventive Care and Screening: Influenza Immunization (NQF#0041)
➢ Pneumococcal Vaccination Status for Older Adults (NCQA measure)
P. 35878 Medicare Shared Savings Program (MSSP)
The Medicare Shared Savings Program (MSSP) presents an important opportunity to promote higher quality and more efficient health care for Medicare beneficiaries. AVAC believes the CMS should engage in a focused, concerted effort to improve access and utilization of adult immunizations as a means of improving the overall health of Medicare beneficiaries.
AVAC commends CMS for maintaining Influenza Vaccination (ACO #14) under the AIM: Better Health for Populations category but are deeply concerned by the proposed removal of and the Pneumonia Vaccination Status for Older Adults measure (ACO #15) in the Medicare Shared Savings Program (MSSP’s).
Monitoring immunization status and reporting of offered and administered immunizations to patients are critical preventive service benchmarks that help to ensure immunizations remain a priority under new payment models and in the forefront of clinical care standards. Reducing the number of missed immunization opportunities, particularly among Medicare beneficiaries, is critical to improving health and reducing the burden of vaccine preventable disease.
The Annual Influenza Vaccination (ACO #14) and the Pneumonia Vaccination Status for Older Adults measure (ACO #15) represent important baseline measures in determining access to influenza and pneumococcal vaccinations and ascertaining where gaps in access to these services may persist.
These two vaccine preventable conditions exact a heavy toll on adults in terms of health and productivity costs and both measures should remain in the MSSP. According to the Centers for Disease Control and Prevention (CDC), an estimated 900,000 Americans get pneumococcal pneumonia each year, resulting in as many as 400,000 hospitalizations and more than 53,000 deaths. Despite the fact that most pneumococcal pneumonia deaths each year are adults, pneumococcal vaccination rates remain inadequate, with only 63 percent of adults over the age of 64 and 22 percent of high-risk adults being vaccinated.4 By contrast, a recent CDC study of flu-associated deaths prevented over a nine-year period from 2005-2006 through 2013-2014 found that nearly 89 percent were in people 65 years of age and older.
However, AVAC also remains concerned that current and new Medicare payment models could threaten access to critical prevention services such as immunization as providers are under increased financial pressure to provide cost efficient care, particularly to medically complex and chronically ill Medicare beneficiaries. AVAC would encourage CMS to closely monitor the potential impact of payment models such as the MSSP on access to critical preventive services, such as immunization. AVAC would like to work with CMS to explore the different payment model programs underway and lift up best practices that expand and improve access to immunization services as well as other lifesaving prevention interventions.
p. 35964 Social Risk Factors
AVAC coalition members are working on projects that seek to identify and enhance our understanding of coverage gaps and are developing pilot programs to test targeted solutions where these disparities currently exist. The proposed rule indicates that CMS is currently reviewing reports by the Office of the Assistant Secretary for Planning and Evaluation (ASPE) and the National Academies of Sciences, Engineering and Medicine on accounting for social risk factors in the Hospital IQR Program. We support the idea of future stratification of IFR QRP data by race, ethnicity, geographic area, sex, and disability on Hospital Compare, as well as on potential future hospital quality measures that incorporate health equity. Additionally, we recommend that the data also be stratified by primary language. Together, this type of data will enable more accurate evaluation in coverage gaps and disparities, particularly among minority and vulnerable populations, and are essential to improving the impact of adult immunization efforts and expanding coverage.
p. 36092 CY2019 MIPS Specialty Measure Sets (Appendix 1)
Opportunities to assess the immunization status of Medicare beneficiaries for should be done by the range of clinicians who care for them, including primary care and specialty providers. Taking advantage of each and every patient encounter to ensure that counseling and education on vaccines, based on their age and health status, and a strong provider recommendation have been found to improve the likelihood of a patient being immunized. Published literature indicates that integrating immunization assessment and additional providers offering these critical preventive services will result in greater opportunities for immunization. The National Vaccine Advisory Committee’s (NVAC) Adult Immunization Standards call for all providers caring for adult patients to assess, recommend, vaccinate or refer, and document vaccinations.6 AVAC greatly appreciates that the proposed rule addressed past concerns that have been raised about the limited number of specialty sets that included adult immunization quality measures. Specifically, the inclusion of Preventive Care and Screening: Influenza Immunization (NQF #0041) and Pneumonia Vaccination Status for Older Adults (NQF# 0043) into a number of primary care and specialty quality measure sets reflects an important advancement that will help ensure better access to immunization services across Medicare providers.
Prioritizing quality measures around immunizations will help close existing measure gaps, improve upon immunization rates and health outcomes for the millions of Medicare beneficiaries. The National Quality Forum (NQF) in its August 2014 report “Priority Setting for Healthcare Performance Measurement: Addressing Performance Measures Gaps for Adult Immunizations”, highlighted ten age specific and composite measure gap priorities that should be addressed.
The proposed rule also seeks to adopt 10 new quality measures into the MIPs program for 2021 payment. AVAC is pleased that one of the new measures is for Zoster (Shingles) Vaccination.
This non-NQF endorsed measure is already in use by the Home Health Value-Based Purchasing Program. AVAC supports broader adoption of a herpes zoster measure across specialty sets to reduce the number of missed immunization opportunities for this debilitating condition. According to the CDC, 27.9 percent of adults age 60 and older reported receiving the herpes zoster vaccine.8 The health and economic burden associated with shingles and its complications are significant. As cited by the CDC, in 2007, the Agency for Healthcare Research and Quality (AHRQ) estimated the average cost of shingles and its complications to be $566 million a year while another study estimated the overall cost could be as high as $1.7 billion a year.
AVAC supports a meaningful core quality measure sets for widespread use to both inform clinical decision making at the point of care and improve quality in the provider setting. CMS has made the alignment of quality measures with the National Quality Strategy (NQS), the CMS Strategic Plan, and other CMS quality reporting and value-based purchasing programs a priority. AVAC fully supports the alignment of reporting mechanisms and believes doing so will strengthen and enhance the development and implementation of adult immunization quality measures.
AVAC was encouraged that the following specialty sets include the following immunization process quality measures for the 2021 payment year:
✓ Family Medicine. Zoster (Shingles) Vaccination; Preventive Care and Screening: Influenza Immunization (NQF# 0041); Pneumonia Vaccination Status for Older Adults (NQF# 0043); and Immunizations for Adolescents (NQF # 1407)
✓ Internal Medicine. Zoster (Shingles) Vaccination; Preventive Care and Screening: Influenza Immunization (NQF# 0041); Pneumonia Vaccination Status for Older Adults (NQF# 0043); and Immunizations for Adolescents
✓ Obstetrics/Gynecology. Preventive Care and Screening: Influenza Immunization (NQF# 0041) and Pneumonia Vaccination Status for Older Adults (NQF# 0043)
✓ Otolaryngology. Preventive Care and Screening: Influenza Immunization (NQF# 0041) and Pneumonia Vaccination Status for Older Adults (NQF# 0043)
✓ Pediatrics. Preventive Care and Screening: Influenza Immunization (NQF# 0041); Childhood Immunization Status (NQF #0038); and Immunizations for Adolescents (NQF # 1407)
✓ Preventive Medicine. Zoster (Shingles) Vaccination; Preventive Care and Screening: Influenza Immunization (NQF# 0041); Pneumonia Vaccination Status for Older Adults (NQF# 0043); and Immunizations for Adolescents
✓ Nephrology. Zoster (Shingles) Vaccination; Preventive Care and Screening: Influenza Immunization (NQF# 0041); Pneumonia Vaccination Status for Older Adults (NQF# 0043); and Immunizations for Adolescents
✓ Oncology. Zoster (Shingles) Vaccination; Preventive Care and Screening: Influenza Immunization (NQF# 0041); Pneumonia Vaccination Status for Older Adults (NQF# 0043); and Immunizations for Adolescents
✓ Infectious Disease. Zoster (Shingles) Vaccination; Preventive Care and Screening: Influenza Immunization (NQF# 0041); Pneumonia Vaccination Status for Older Adults (NQF# 0043); and Immunizations for Adolescents
✓ Rheumatology. Preventive Care and Screening: Influenza Immunization (NQF# 0041); Pneumonia Vaccination Status for Older Adults (NQF# 0043)
✓ Geriatrics. Zoster (Shingles) Vaccination; Preventive Care and Screening: Influenza Immunization (NQF# 0041); Pneumonia Vaccination Status for Older Adults (NQF# 0043); and Immunizations for Adolescents (NQF # 1407)
✓ Skilled Nursing Facility. Zoster (Shingles) Vaccination; Preventive Care and Screening: Influenza Immunization (NQF# 0041)
AVAC was, however, disappointed that the final rule did not include adult immunization quality measures in a few key specialty areas who care for chronically ill patients at-risk of serious complications from vaccine preventable illness. The Advisory Committee on Immunization Practices (ACIP) includes age-based, as well as condition-specific recommendations for adult vaccination. For pregnant women, ACIP recommends a Tdap vaccination. We are pleased that efforts to develop a composite Tdap/influenza measure for pregnant women has completed testing and is now under review by the National Committee for Quality Assurance (NCQA). AVAC looks forward to further dialogue your agency on this topic as it moves forward.
In addition, patients living with chronic conditions such as heart disease and diabetes are at a significantly higher risk of complications and death from influenza and pneumonia. The CDC has reported that in 2013 only 21.2% of adults in this group had received a pneumococcal vaccination, and this number has remained unchanged for at least a decade.10 Individuals with diabetes are at increased risk for hepatitis B infection. As such, the ACIP recommends hepatitis B vaccination for all patients with diabetes age 6011 and under as well as other at-risk patients, such as those living with HIV/AIDS and chronic kidney disease.
We strongly encourage CMS to add the following immunization quality measures into these specialty measure sets:
Endocrinology. Zoster (Shingles) Vaccination; Preventive Care and Screening: Influenza Immunization (NQF# 0041) and Pneumonia Vaccination Status for Older Adults (NQF# 0043).
Cardiology. Zoster (Shingles) Vaccination; Preventive Care and Screening: Influenza Immunization (NQF# 0041) and Pneumonia Vaccination Status for Older Adults (NQF# 0043).
General Surgery. Preventive Care and Screening: Influenza Immunization (NQF# 0041) and Pneumonia Vaccination Status for Older Adults (NQF# 0043).
Skilled Nursing Facility. Pneumonia Vaccination Status for Older Adults (NQF# 0043)
We appreciate this opportunity to share our perspective on this proposed rule. Please contact an AVAC Coalition Manager at (202) 540-1070 or info@adultvaccinesnow.org if you wish to further discuss our comments. To learn more about the work of AVAC visit www.adultvaccinesnow.org.
Sincerely,
Alliance for Aging Research
American Immunization Registry Association (AIRA)
American Pharmacists Association
Asian Pacific Islander American Health Forum
BIO
Dynavax
Every Child By Two (ECBT)
Families Fighting Flu
GSK
Hepatitis B Foundation
Hep B United
Infectious Diseases Society of America (IDSA)
Immunization Action Coalition
Immunization Coalition of Washington DC
Medicago
Merck
National Association of County and City Health Officials (NACCHO)
National Black Nurses Association
National Foundation for Infectious Diseases (NFID)
Novavax
Pfizer
Sanofi
Seqirus
Takeda Vaccines, Inc.
The Gerontological Society of America
Trust for America’s Health